Nasal aerosol inhalation is increasingly used in intensive care units (ICUs). In pediatric wards in the United States, 75% of patients receive nasal aerosol inhalation therapy, while the rest use traditional nebulization methods.
For patients with severe hypoxemia, routine use of high-flow nasal oxygen therapy (HFNC) can help improve oxygenation and ultimately avoid intubation or reintubation. HFNC nasal aerosol inhalation combines the advantages of HFNC and aerosol therapy.
Since 2008, in vivo/in vitro studies have explored the many factors that affect the effect of HFNC aerosol inhalation and the clinical effectiveness of the nasal aerosol inhalation route.
In 2018, Braunlich et al. studied 26 patients with stable chronic obstructive pulmonary disease (COPD) and found that HFNC (TNI Medical AG, Germany) combined with an airflow of 35 L/min had a similar bronchodilator effect compared with inhalation of 2.5 mg salbutamol and 0.5 mg ipratropium bromide via a small volume jet nebulizer (JN) (P=0.5). Similarly, ReMiniac et al. studied 25 stable patients with reversible airflow obstruction and found that HFNC (Airvo2, New Zealand) combined with a vibrating mesh nebulizer (VMN) (Aerogen Solo, Ireland) nebulization therapy had a comparable improvement in forced expiratory volume in the first second (FEV1) compared with inhalation of 2.5 mg salbutamol via a mask JN (P=0.11). Madney et al. compared the bioavailability of salbutamol after inhalation of JN or VMN combined with HFNC at a flow rate of 5 L/min in a crossover randomized controlled trial of 12 patients with stable COPD. The results showed that the urinary salbutamol excretion in the VMN group was twice that in the JN group after 30 minutes and 24 hours (P<0.05).
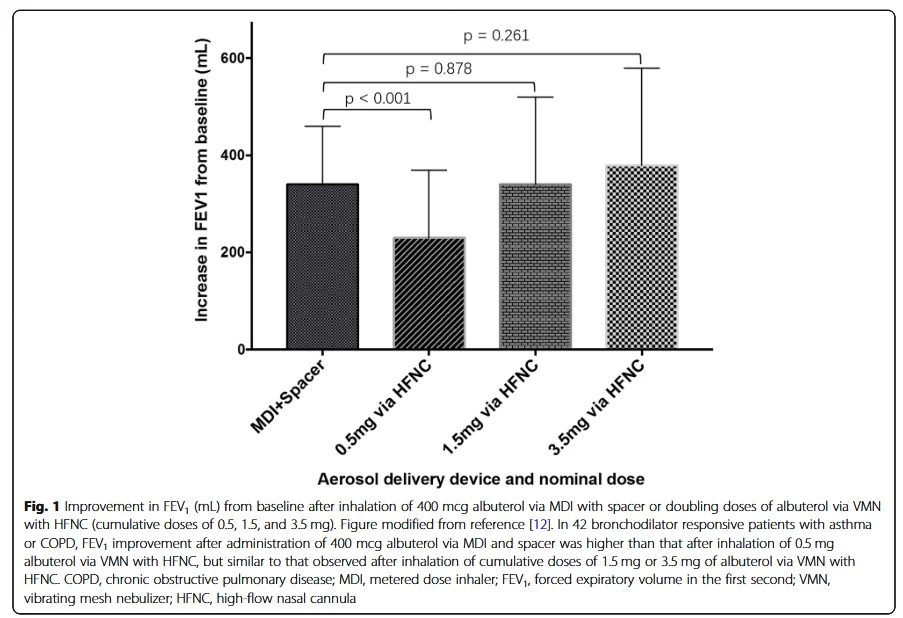
In the United States and Europe, the labeled dose of albuterol solution is 2.5 mg. Li et al. conducted a dose-response analysis in 42 patients with stable asthma or COPD. It is known that these patients respond to 400 mcg of albuterol inhaled via a metered dose inhaler (MDI) combined with a nebulizer (SPACER). The subjects received increasing doses of albuterol inhaled via HFNC combined with VMN at an airflow of 15 to 20 L/min. When the cumulative dose of albuterol reached 1.5 mg, the improvement in FEV1 with HFNC combined with VMN was comparable to that with MDI combined with SPACER (P=0.878) (Figure 1).
Inhaled prostacyclin is a pulmonary vasodilator that can be used to treat patients with pulmonary hypertension and/or refractory hypoxemia who require mechanical ventilation. Two small retrospective studies found that adult patients with pulmonary hypertension and refractory hypoxemia had improved oxygenation after inhaled prostacyclin with 40 L/min of HFNC airflow. Bedside individualized titration of HFNC airflow based on the patient's response to prostacyclin was effective in reducing mean pulmonary artery pressure compared with a constant HFNC airflow. Future prospective studies with larger sample sizes are needed to validate this finding.
In 2015, Morgan et al. conducted a study on 5 infants and found that children with acute bronchiolitis and dyspnea did not respond to JN nebulization treatment via mask. However, after inhaling salbutamol via HFNC combined with VMN, the infants showed good comfort, which indicates that the combination of salbutamol and HFNC can benefit patients. Compared with salbutamol nebulization via mask combined with VMN, the heart rate of patients in the HFNC combined with VMN group was significantly increased (P<0.001). The reason for the significant increase in heart rate may be that the dose of salbutamol inhaled via HFNC combined with VMN is higher. Compared with mask combined with JN, the comfort and satisfaction of patients after salbutamol nebulization via HFNC (about 8 L/min) combined with VMN was significantly improved. A retrospective analysis of 39 children with status asthmaticus who failed to receive JN nebulized albuterol (10 of whom had severe acidosis, pH < 7.30) found that the maximum airflow of intermittent albuterol administration via HFNC was 1.0 (0.8-1.1) L/kg/min, which was one of the reasons for avoiding endotracheal intubation.
The standard airflow for nebulized bronchodilator inhalation via HFNC is 15-35 L/min for adults and 1 L/kg/min for children, and the clinical effect produced at this time is similar to that of traditional nebulizer devices. The efficiency of nebulized inhalation in critically ill patients needs further study.

中国大陆
简体中文
United Kingdom
English